Part of my Life in the Lab Series.
Here I summarise what each of my scientific roles have entailed.
As a Microbiology Technician, what does the day entail? Continue reading
Drawing from over 20 years of industry experience, gain valuable insights and expert perspectives from a trusted microbiology quality assurance leader and internal auditor. Bonus AI and IT skills.

Here I summarise what each of my scientific roles have entailed.
As a Microbiology Technician, what does the day entail? Continue reading

My work called for presenters on a wide range of topics, both work and non work related. I put my hand up to deliver a talk on something drawn from my background as a scientist. After some thinking I came up with “Quality Assurance – How to Apply Scientific Principles to Troubleshooting”. I thought that would be a good topic as that’s the main thing I do in my current role and I did a lot of it in my old roles. I thought I would be able to impart knowledge to help others examine and solve their problems. Project mangers might find it useful. Software developers might find it useful. Athletes might find it useful. Continue reading
There was an advert for a science communicator / project manager this week. The twitter post said must love science (*tick*) and have skills (*tick*). I checked it out. The role was skewed towards science communication and requires someone with a history of producing media reports, blogging, liaison with the science industry etc. Enthusiasm alone would not cut it, though it would make the role a challenge, and that’s what I’m here for – challenges.
One requirement was to be able to name at least 10 science organisations in Australia. Hmm, thought I cannot do that. That’s embarrassing. So, here’s what I could name off the top of my head and then here’s what I could find using the great advertising machine known as Google.
Nine’s not bad, though I should know at least 20.
After four, I felt I was scraping the bottom of the Google barrel. I soon discovered that while many about pages may list the organisation name in the header meta, the names were missing from the body of the page. I also found that in lots of articles about science produced by Australia, the organisation producing the science was not named. Not how you build brand recognition.
Related to this, I could name lots of science Australia has produced or is working on. The various inventions reportedly to come out of Australia, (Hills hoist, the ute, the stump jumper), stomach ulcers being caused by Helicobacter pylori, matter transport/Quantum entanglement, eradicating rats by using dogs from an Island off the coast of Warrnambool (awww, Oddball died in February. I’ve also merged this with Macquarie Island where dogs were used to eat all the rabbits, rats and mice – Oddball was a fox eaterupperer), wi-fi (CSIRO), the joint US-Australian military research project called Hypersonic International Flight Research Experimentation (HIFiRE) testing hypersonic engines, the people developing organs in a petri dish, various cancer research and I’m sure I could come up with more.
On the topic of Australian science, have a read of Australia’s National Science Statement – 2017. It states science means “Natural, physical and life sciences, including medical and health sciences, mathematics, engineering and technology‑related disciplines.”
That’s not a proper description. Science is the methodical and rigorous study of phenomena to understand them and the application of such methods and results to produce things. The results can be knowledge, TV’s, vaccines, better car tyre rubber, ducks that go woof, less harrowing cancer treatments, key hole surgery, the “discovery” that fat is turned to water and CO2 and expelled in order to lose weight – even if it is obvious, it needs proving.
Making sure I had some idea my above description in bold was more or less correct, I checked our a dictionary and it said “the intellectual and practical activity encompassing the systematic study of the structure and behaviour of the physical and natural world through observation and experiment.” Yup – within acceptable error limits.
From Jan 1, 2017, the PIC/S GMP guide for Medicinal Products V13 will be in effect. In Australia, the TGA requires version 9 is used, though rumour has it, version 13’s going to be adopted soon. If such proposals as this one, which is mainly concerned with herbal “medicine” from the Complimentary Healthcare Council are ratified, then all future PIC/s updates will automatically apply to Australian manufacture. Something to keep an eye on.
Compliance wise adopting PICS/s 13 over 9 is not too big as deal as the PIC/S guide is based on old ICH Q7A guidelines dating from August 2001 and the right and wrongs do not change much over time unless something major takes place. What manufacturers will need to pay attention to are the changes between version 9 and 13, namely:
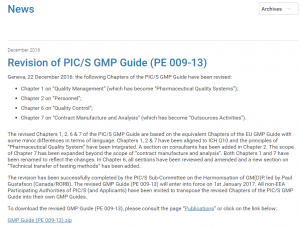
Screen shot of PIC’s news item
Link to guide. (possibly dead) – Google search will find it for you.
Based on the contents of V9, things to review:
You should already be in control of the above areas as otherwise, you’d be having and uncomfortable 3rd party audit experience. The overall principles do not change, just the fine-print.
Annex 1 deals with the manufacture of sterile medicinal products, so not much impact to us there. If you are performing risk assessments and utilising ISO9001, QRM should already be familiar to you.
Did you find this informative or useful? Please consider a small donation so I can expand and improve on what I deliver.
Evernote seems to be a powerful, extensible cloud based application. I am curious if anyone in the group uses it in their lab and how do they use it, for what purpose and how well does it work for your needs?/”
I’d not advise it. Besides a vendor audit to ensure availability of the system and backups you’d need to ensure Evernote data cannot be obscured or changed, make sure time, date and user stamps are in place and the data integrity is maintained for the duration of the retention period.

Like any other situation where you wish to convey accurate and important information, documentation/notes/written examples rule.
Attending a job interview is a very unnatural situation. Once in a role, you have access to documentation, procedures, reference materials, pre-made presentations etc to get your point across. In interviews you have your memory, your dress sense and your people skills. To add confusion to the mix, recent studies are showing memory can be flawed so how am I to know an example I am providing actually happened, or was directly related to me? Notes! That is how! Continue reading
This is something I tend to be weak on when it comes to answering in an interview (it does not come up much). Hence this post to begin to get things ticking over in my brain.
Continuous improvement (CI) is something I’ve routinely done as I’ve updated documents, mapped business processes or followed procedures. I’ve not followed any official 6 sigma methodology (also known as Lean, Agile, Kaizen) as such, though I have the basics if this in my survival kit and ask why 5 times.
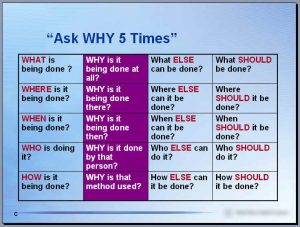
Ask Why 5 Times – pinned to the wall of my cubicle/office
CI is a method for identifying opportunities for streamlining work and reducing waste. Continue reading

As you may have read, I’m an INTJ (or Architect) and a DiSC Panther. As part of recent communication with a recruiter, I’ve partaken in yet another personality test. This one pegs me as an Administrator. Keeping in mind a certain amount of salt needs to be taken with such tests as these tests are all made up and personalties are fluid.
An Administrator is precise and reserved. They want to do things right and pay attention to detail. Had this test produced the same output as my DiSC appraisal, I’d most likely have been a Driver (they want to take charge in order to succeed and win) with a smattering of Promoter (wanting to influence others and inspire them to act). As it is, I’m, 38% Driver, 50% promoter and 100% Administrator according the latest test. Continue reading

Why would anyone want a Quality Management System, or QMS? To quote the ISO, “It helps businesses and organisations be more efficient and improve customer satisfaction.” The document that details such a system is ISO 9001 (2015) Quality Management Systems. A large part of this ISO reads as a business plan – improve performance; sustainable development; identify opportunities / enhance customer satisfaction etc. Overall, the ISO employs the approach of Plan-Do-Check-Act and requires risk-based thinking. Continue reading
While larger companies nowadays use validated systems such as Trackwise, SAP modules and the like for following-up on their CCPs, deviations, CAPAs etc., in GMP inspections of smaller companies I usually encounter some sort of electronic Access- or Excel-based lists that are used (typically by QA) for this purpose, i.e. entering cases, assigning event ID’s, and supervising the progress of investigations / implementation measures until approval / close-out.
These lists a quite critical, especially when many events have to be dealt with, a.o. because of the risk that certain events might get ‘forgotten’.
Nevertheless, I see quite often that little is done to ensure that entries in these lists are correct and uptodate. Another issue is controlled handling of hardcopies of these lists which often, not astonishingly, are outdated as soon as they come out of the printer.
Any ideas what it makes challenging to deal with these lists? What are proven good practices worthwhile to share?”
Just like for word, Excel has a revision tracking feature.
There are ways to log changes to tables data in Access, but none are particularly robust.
It all boils down to using the right tool for the job. Is Excel or Access a suitably regulatory compliant solution or do you need to use something else?
The last line of the my comment sums things up nicely.