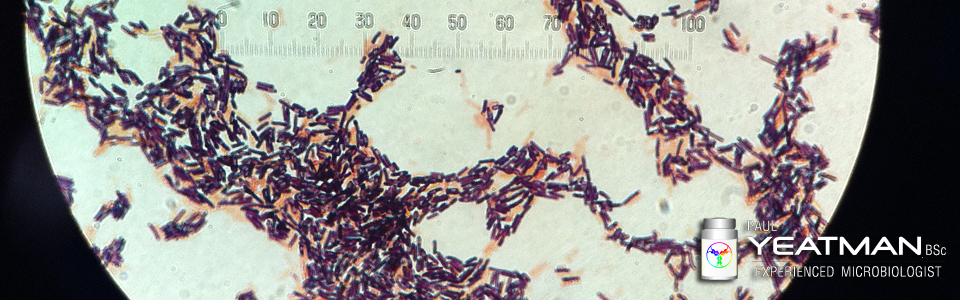
Having worked in sterile production environments where contamination control was vital, there were zero exceptions to following the established procedures. Exceptions would invariably lead to unacceptable outcomes. With regards to lock-down and quarantine in relation to SARS-COV-2, there are many exceptions and deviations from the requirements. That will only lead to….you guessed it, an unacceptable outcome. With this in mind, I present to you a brief article on contamination control in sterile production environments. Continue reading