As of March 4 2020, 16 of the last 50 recalls by the FDA for pharmaceutical products were due to microbial contamination or a lack of sterility assurance. Another 27 were due to impurities with 5 more due to mislabeling. Looking at TGA recalls for 2019, around half of the medicine recalls due to contamination were the result of microbial contamination. Continue reading
Category Archives: How I Benefit You
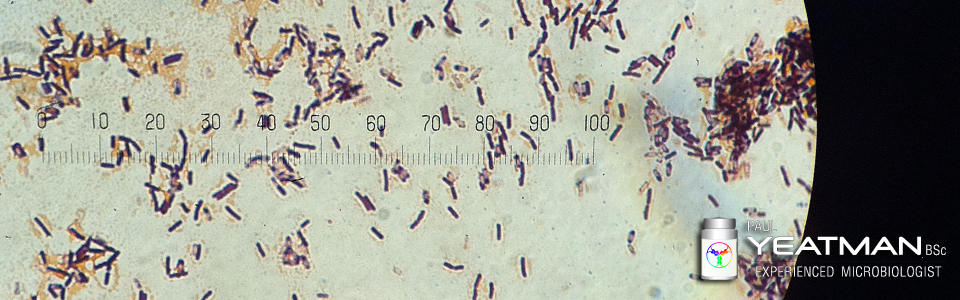
What is Data Trending and How To Identify, Action and Report Trends
The following is an article I placed on LinkedIn and was written as part of my Developing My Writing While Helping Others series.
Paul Yeatman is a microbiologist with over 15 years’ experience in documentation, validation and running investigations in TGA and FDA regulated environments. He has a strong interest in process improvement, documentation, training and developing others. From 9-5 Paul investigates and solves software problems. By night he works on his science chops. He has an arty streak, runs several blogs and enjoys communicating his experiences and knowledge in arenas such as this. Continue reading
Controlled Documentation. Identifying what goes into a Policy, a SOP and an OI
The following is an article I placed on LinkedIn and was written as part of my Developing My Writing While Helping Others series.
I am a microbiologist with over 15 years’ experience in the pharmaceutical realm. I have a strong interest in regulatory compliance, documentation and developing others. Recently I have been working closely with data security. I have an arty streak, have developed and delivered training and have an affinity for computers. I ride bicycles…a lot.
Controlled Documentation. Identifying what goes into a Policy, a SOP and an OI Continue reading

Ten GMP Self Inspection or Internal Audit Considerations
Posted as a LinkedIn article on 20190718
Preamble
I am a microbiologist with over 15 years’ experience in the pharmaceutical industry supporting the manufacture of liquids, creams, ointments and tablets. I have a strong interest sterile manufacturing, leading and developing others. Recently I have been working closely with data security. I have an arty streak, an affinity for computers and ride bicycles…a lot.
Introduction
Good Manufacturing Practice in Australia uses recommendations presented in the PIC/s Guide to GMP (PE009-13). The section on Quality Management states, “there is a procedure for self-inspection and/or quality audit, which regularly appraises the effectiveness and applicability of the Pharmaceutical Quality System”. Chapter 9 deals entirely with Self Inspection and lists 3 points – not entirely helpful. Continue reading
Developing My Writing While Helping Others
I am a microbiologist with over 15 years’ experience in the pharmaceutical realm. I have a strong interest in regulatory compliance and developing others. Recently I have been working closely with data security. I have an arty streak, have been a work place trainer and have an affinity for computers.
Lately I’ve been thinking of ways to share my knowledge with others outside of my cycling and science blogs and have decided to write 12 LinkedIn posts over 12 months. I will limit the post size to between 500 and 1000 words (1-2 A4 pages). Continue reading
Review of Draft Standard: AS 2828.2 Health records, Part 2: Digitized health records
One way I keep myself up to date with developments within laboratories and related areas is by reviewing draft standards. This keeps me appraised of the current state of affairs, keeps my documentation audit skills fresh and potentially allows me to contribute to the content of standards. For this draft standard, I have some knowledge of IT and IT security so am able to critically review the draft standard and offer comment.
Notes: refer to the conditions for comment stated towards the beginning of the draft standard.
DR AS 2828.2 Health records, Part 2: Digitized health records Continue reading
Review of Draft Standard: AS 2243.2 Safety In Laboratories – Part 2: Chemical Aspects
One way I keep myself up to date with developments within laboratories and related areas is by reviewing draft standards. This keeps me appraised of the current state of affairs, keeps my documentation audit skills fresh and potentially allows me to contribute to the content of standards.
Notes: refer to the conditions for comment stated towards the beginning of the draft standard.
Update 20190103: After proceeding to submit comments, I observed page numbers were required. That is important to note for future reviews. Page numbers added.
DR AS 2243.1 Safety In Laboratories – Part 2: Chemical Aspects Continue reading
Review of Draft Standard: AS 2243.1 Safety In Laboratories – Planning and Operational Aspects
One way I keep myself up to date with developments within laboratories and related areas is by reviewing draft standards. This keeps me appraised of the current state of affairs, keeps my documentation audit skills fresh and potentially allows me to contribute to the content of standards.
Here I step through the draft standard making comments. Where a comment is answered later in the standard, I go back to my original comment and make notes. An uncommented comment is potentially worthy of becoming an official comment on the standard.
Update 20190103: page nubmers added.
Section 1 Continue reading
Life in the Lab – Microbiology Team Leader
Part of my Life in the Lab Series.
Here I summarise what each of my scientific roles have entailed.
As a Microbiology Team Leader (non sterile laboratory), what does the day entail?
08:30 hrs – Start shift Continue reading
Life in the Lab – Quality Assurance Microbiologist
Part of my Life in the Lab Series.
Here I summarise what each of my scientific roles have entailed.
As a Quality Assurance Microbiologist, what does the day entail? Continue reading