The following is an article I placed on LinkedIn and was written as part of my Developing My Writing While Helping Others series.
I am a microbiologist with over 15 years’ experience in the pharmaceutical realm. I have a strong interest in regulatory compliance, documentation and developing others. Recently I have been working closely with data security. I have an arty streak, have developed and delivered training and have an affinity for computers. I ride bicycles…a lot.
Controlled Documentation. Identifying what goes into a Policy, a SOP and an OI
Introduction
Within a regulated environment, there are multiple levels of documentation. The purpose of such documentation is to ensure work is carried out in a legal, regulatory complaint and consistent manner. These documents must be clear, not open to interpretation and justified in their content. There are three levels of controlled documentation. Policies, Standard Operating Procedures (Procedures) and Operator (or work) Instructions. Each type of document serves a specific purpose and needs to contain relevant information. Here I discuss the three levels of documentation and identify the purpose of each and the information each should contain.
The Three Types of Documentation
Within a controlled documentation system, a hierarchy of documents is advantageous. In the companies I have worked for, there have typically been three. Policies, Standard Operating Procedures and Operator (or work) Instructions. Each document has a specific purpose and contains specific information.
The Policy
A high level document that generalises what goes on. These documents contain regulator information (or references to the information), internal polices, rules and principles and why they are in place.
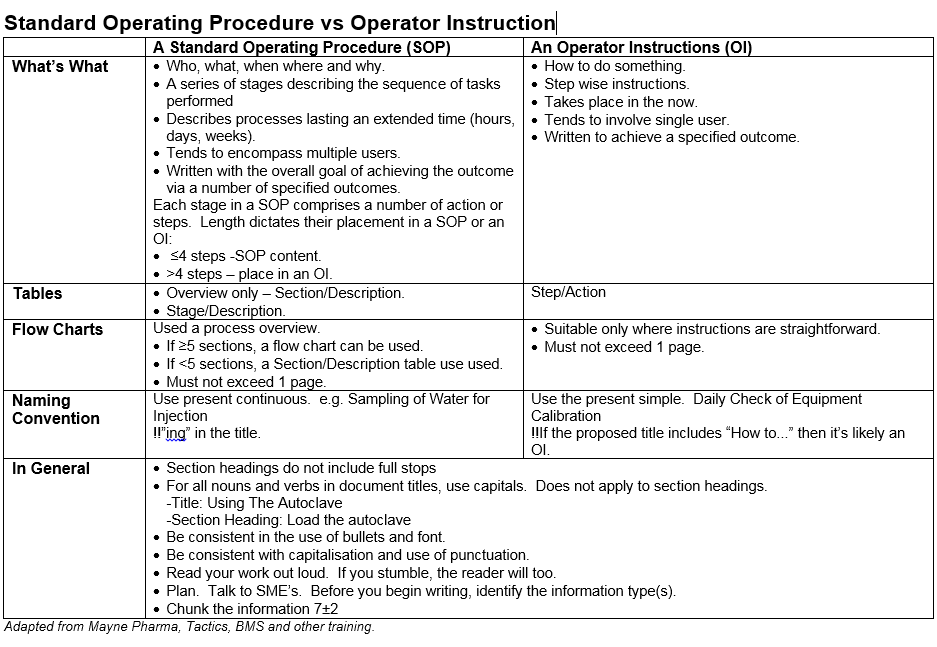
The Standard Operating Procedure (SOP)
This document describes overarching areas, such as Bacteriology, the Viable Environmental Monitoring Program, Site Validation, and Sterility Testing.
A SOP details a series of tasks that takes place over time with an identifiable goal.
As an example, a bacteriology SOP would bring together everything to do with this subject including plating, identification, maintaining a culture collection with the aim (perhaps) to allow staff to identify and maintain microbes.
The language in this document should be clear and not open to interpretation.
The Operator Instruction (OI)
This is the lowest level of documentation and describes a specific process that usually can fit on a page or two and has a specified outcome. Examples based on the subjects listed in the SOP section above include for Bacteriology: Perform a Gram Stain, for the Viable Environmental Monitoring Program: Operation of the MAS100 Air Sampler, for Site Validation: Validate A New Incubator and for Sterility Testing: Sanitise the Isolator with Hydrogen Peroxide.
Using the Perform a Gram Stain example, this would detail the step by step process for taking a colony off a plate and staining it using the Gram stain method.
What Information goes in each document
A policy explains the reasons behind a process.
A SOP provides the who, what, where when and order of something. If a process takes more than a day (generally) then a SOP is the appropriate document to write. A SOP might be used by more than one person at a time.
An OI is more time limited than a SOP and the task the OI details is normally conducted by a single person at a time. Steps that may be generalised in a SOP are specifically stated within a OI. Do this. Do that. Do this other thing.
Document Structure
Like any well planned writing, a logical structure is important. All documents should have a header and footer containing such information as writer, verifier and approver, approval and expiry dates, document number, title, page number and total pages.
A policy could be set out as follows:
Purpose, Background, Roles and Responsibilities, Overview, Listing of related polices, Glossary, References.
A SOP could be set out as follows:
Purpose, Who uses the policy, EHS considerations, Before you begin, Procedure, Additional information, Glossary, References.
An OI could be set out as follows:
Purpose, Who uses the OI, Before you begin, Overview, Instructions.
Other Considerations
Templates
Useful for providing consistency of format and providing examples of acceptable content. A template ensures consistent document structure and formatting of titles, headings, body and table content.
Scheduling and Planning
Used alongside document validity dates. Enable the documentation controller and users to proactively schedule document review and update. Planning is important so the document is updated without rushing (i.e. the week before it is due to expire).
Who Writes the Documents
The subject matter expert (SME) is the best writer. Don’t be afraid of delegation however. A technical writer can be used provided the document is written in consultation with the SME.
Who Reviews and Approves Documents
The reviewer should be a peer or someone in the management chain for the writer as they should also have knowledge of the process being documented. The reviewer looks for content accuracy, correct grammar and correct references. Ideally, the writer does the review and a verifier is used to confirm the work of the reviewer. This reduces work for both the writer and the verifier as the document does not have to keep going back and forth between the two.
The Approver will ideally be the manager of the department responsible for the process being documented.
Change History
It is very important to track changes and document the reasoning behind changes. Helps an auditor to identify and approve changes. In my view, this section can never contain too much information.
Training
Staff need to be trained to follow SOP’s and OI’s and when a significant change to a SOP or OI is made, your staff need to be trained in the changes.
Standard Procedure for Documents
In order to produce consistent documentation with sensible content in a consistent format, procedure for doing this is helpful and within a regulated environment, a necessity.
Conclusion
You will now be familiar with the three main types of controlled documents: Polices, SOPs and OI’s, when they are suitable for use and what sort of information each type of document should contain.
Appendix
Did you find this informative or useful? Please consider a small donation so I can expand and improve on what I deliver.