You are welcome to send me emails offering me your web development, advertising or SEO services. Sending such emails is subject to the following legally binding conditions.
Auditor – Investigator – Trainer – Validator
I specialize in microbiology quality assurance and control, and get a kick out of assisting pharmaceutical and FMCG companies in streamlining their processes and making them audit ready.
My goal is to ensure timely product releases while maintaining an audit-ready state, thereby reducing OOS incidents, minimizing audit observations, and ultimately saving costs.
Keeping up with the latest developments in the pharmaceutical and food manufacturing industries is a passion of mine. I enjoy sharing my knowledge and staying abreast of the evolving rules, regulations, and guidelines.
A little about my 20+ year career
I quickly progressed to Senior Microbiologist, leading a team of 12-15 individuals. I then transitioned into project-based work as a Quality Assurance Microbiologist. Later I moved up to Microbiology Team Leader position. Due a role redundancy in 2012 derailing things, I decided to create this website to share my knowledge and experience.
During Covid I secured a Quality Coordinator role with a dairy producer in regional Victoria. From October 2022 to January 2025, this included a secondment to a sister site in Melbourne as a Quality Manager.
I have worked in both generic sterile (FDA) and novel non-sterile production (TGA/EU) plants, where I have conducted equipment and process validations, staff training, audits, and honed my passion for documentation.
My area of expertise includes USP1116, ISO13408, ISO14644, ISO14698, ISO17025, ISO9000, PIC’s, HACCP and SQF. I am an adept and trained internal auditor and a certified trainer of small groups.
Three words that my best friend uses to describe me:
- loyal
- dedicated
- honest
Another reason why you should add me to your team:
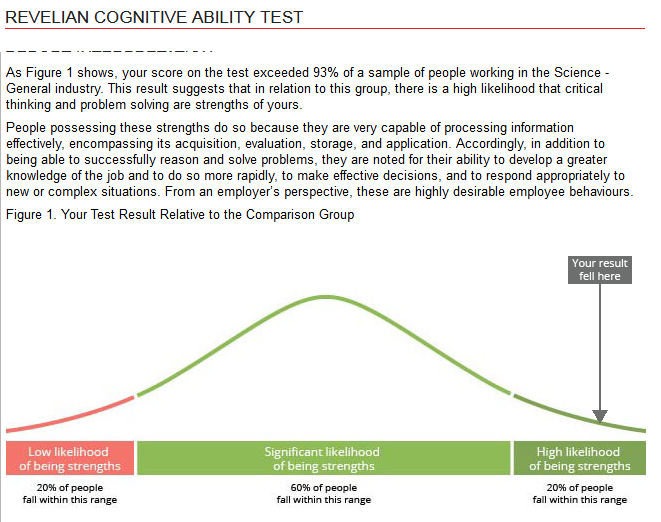
Regarding my critical thinking and problem solving abilities, I am in the top 93% of a comparison group of scientists.
Value Added Components
As well as my Bachelor of Science, I have a Diploma in graphic design, which emphasizes visual communication, as well as certification in training small groups. I have also accumulated valuable experience working as a second-level technical support engineer in the IT industry for several years. At home I host my own LLM AI’s, mail, media and web servers for fun.

