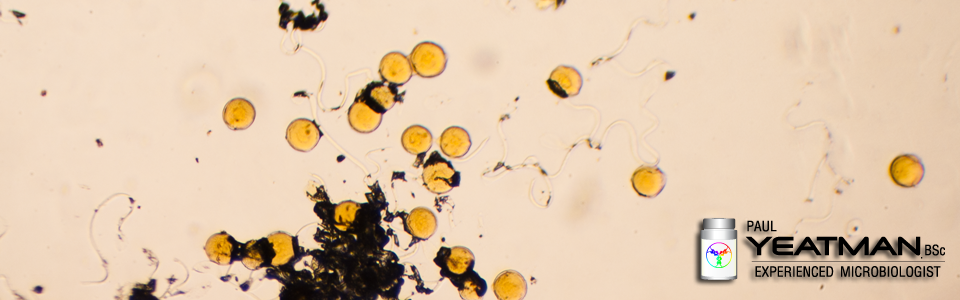
As of March 4 2020, 16 of the last 50 recalls by the FDA for pharmaceutical products were due to microbial contamination or a lack of sterility assurance. Another 27 were due to impurities with 5 more due to mislabeling. Looking at TGA recalls for 2019, around half of the medicine recalls due to contamination were the result of microbial contamination.
It is vitally important that your products are fit for purpose. What is on the label must be in the product. Nothing more. Nothing less. Quality assurance goes a long way and with the correct processes in place, the number of potential batch recalls should be zero.
With regards to microbial contamination, I am updating my “Microbiology for Non-Microbiologists” presentation and will shortly be providing an abridged version online. Stay tuned.
Did you find this informative or useful? Please consider a small donation so I can expand and improve on what I deliver.